Abstract

Background: Until now, myeloid malignancies are classified according to the revised 4th edition of World Health Organization Classification of Haematolymphoid Tumours, published in 2017 (WHO 2017). During the last years, substantive work has been done in the field of genetics, leading to dynamic changes with respect to defining specific sub-entities. Thus, the up-coming 5th edition of WHO Classification (WHO 2022) emphasizes a genetic basis for defining diseases. Amongst other changes, the blast cut-off between MDS and AML with defining genetic abnormalities (DGA) is largely abandoned. In parallel to the WHO 2022, the International Consensus Classification (ICC) sets the blast cut-off for AML-DGA to 10%, while cases with 10-19% blasts without DGA are assigned as a new category MDS/AML.
Aim: Evaluate the impact of the up-coming WHO 2022 guideline on the classification of AML and MDS patients and quantify differences in disease categorization compared to ICC.
Methods: 1451 non-therapy-related cases with MDS or AML diagnosed according to WHO 2017 were included. Bone marrow samples were analyzed by cytomorphology, immunophenotyping, cytogenetics, and whole genome (median coverage 100x) and transcriptome (50 Mio reads) sequencing.
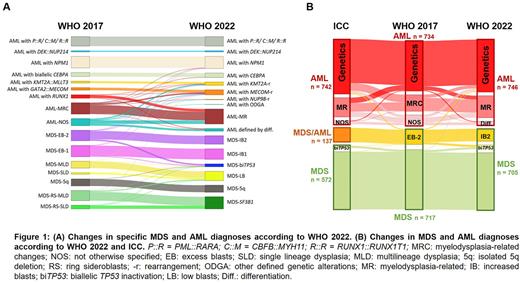
Results: 746 patients were diagnosed as AML according to WHO 2022. Overall, the group of AML-DGA (excluding MR) remained similar to the WHO 2017 with 65% but changed its composition. Major additional contributors were AML with KMT2A-r (n=44) now including 18 cases (41%) with a different partner gene but MLLT3, AML with MECOM-r (n=64) now including 28 cases (44%) not comprising GATA2, and the newly recognized categories AML with NUP98-r (n=5) and AML with other DGA (n=1, KAT6A::CREBBP). In addition, 8 further cases were classified as AML with mutated NPM1, and 5 cases as AML with mutated CEBPA. In contrast, the now abandoned AML with mutated RUNX1 was mainly re-classified as AML-MR (37/48). AML-MR increased from 22% as defined as AML-MRC by WHO 2017 to 28% in WHO 2022. The largest contributor to this increase were mutations in the defining genes solely leading to classification as AML-MR in 44% (92/210) of cases. Notably, cyto- and molecular genetics without medical history were sufficient for AML-MR classification in all but one patient. Complementary to these findings, the morphologically defined subgroups were substantially reduced from 13% AML-NOS to 5% AML with differentiation. 705 patients were diagnosed as MDS according to WHO 2022. The largest changes included the newly defined MDS-biTP53 which was mainly composed from MDS-EB (30/40; 75%) (Fig. 1A).
When considering the main diagnoses in comparison to the WHO 2017, reclassification from MDS to AML according to WHO 2022 was a rare event affecting <1% of cases. In total, 12 MDS samples, 8 of them EB-2, were upstaged to AML based on DGA (MECOM-r: n=5; KMT2A-r: n=1; NPM1: n=6). According to ICC, the cohort would comprise 742 AML, 572 MDS, and 137 MDS/AML cases leading to a reclassification at this level in 10% of cases mainly due to the new ICC category MDS/AML which largely corresponded to MDS-EB2 according to WHO 2017 and to -IB2 and partly -biTP53 according to WHO 2022. In addition, 8 former MDS-EB-2 cases were categorized as AML according to ICC criteria (NPM1: n=4; CEBPA: n=4). The overlap of cases upstaged to AML according to ICC and WHO 2022 were 4/16 patients due to NPM1 mutations and ≥10% blasts (Fig. 1B).
Conclusions: The new classifications consistently follow the idea of a more genetics-based definition substantially reducing purely morphologically defined AML, introducing new genetic subgroups, and making a comprehensive genetic analysis mandatory for diagnosis of AML and MDS. Basic concepts of classification are similar between WHO 2022 and ICC. However, differences in the exact diagnostic criteria lead to non-comparable diagnoses in a subset of patients. While this can be explained within the new ICC category MDS/AML, a small number of patients (~ 1%) will be differently classified as AML or MDS based on the phrasing of the definitions. It is beyond the scope of our study to objectively give preference to one classification over the other. Nevertheless, it is predictable that the parallel usage of two different classifications would confuse the diagnostic language for physicians and patients. A unified commonly accepted classification is essential for comparability of diagnostic data in- and outside of clinical studies.
Disclosures
Huber:MLL Munich Leukemia Laboratory: Current Employment. Baer:MLL Munich Leukemia Laboratory: Current Employment. Hutter:MLL Munich Leukemia Laboratory: Current Employment. Dicker:MLL Munich Leukemia Laboratory: Current Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Pohlkamp:MLL Munich Leukemia Laboratory: Current Employment. Kern:MLL Munich Leukemia Laboratory: Current Employment, Other: Ownership. Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Ownership. Hoermann:MLL Munich Leukemia Laboratory: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal